Li-air Batteries
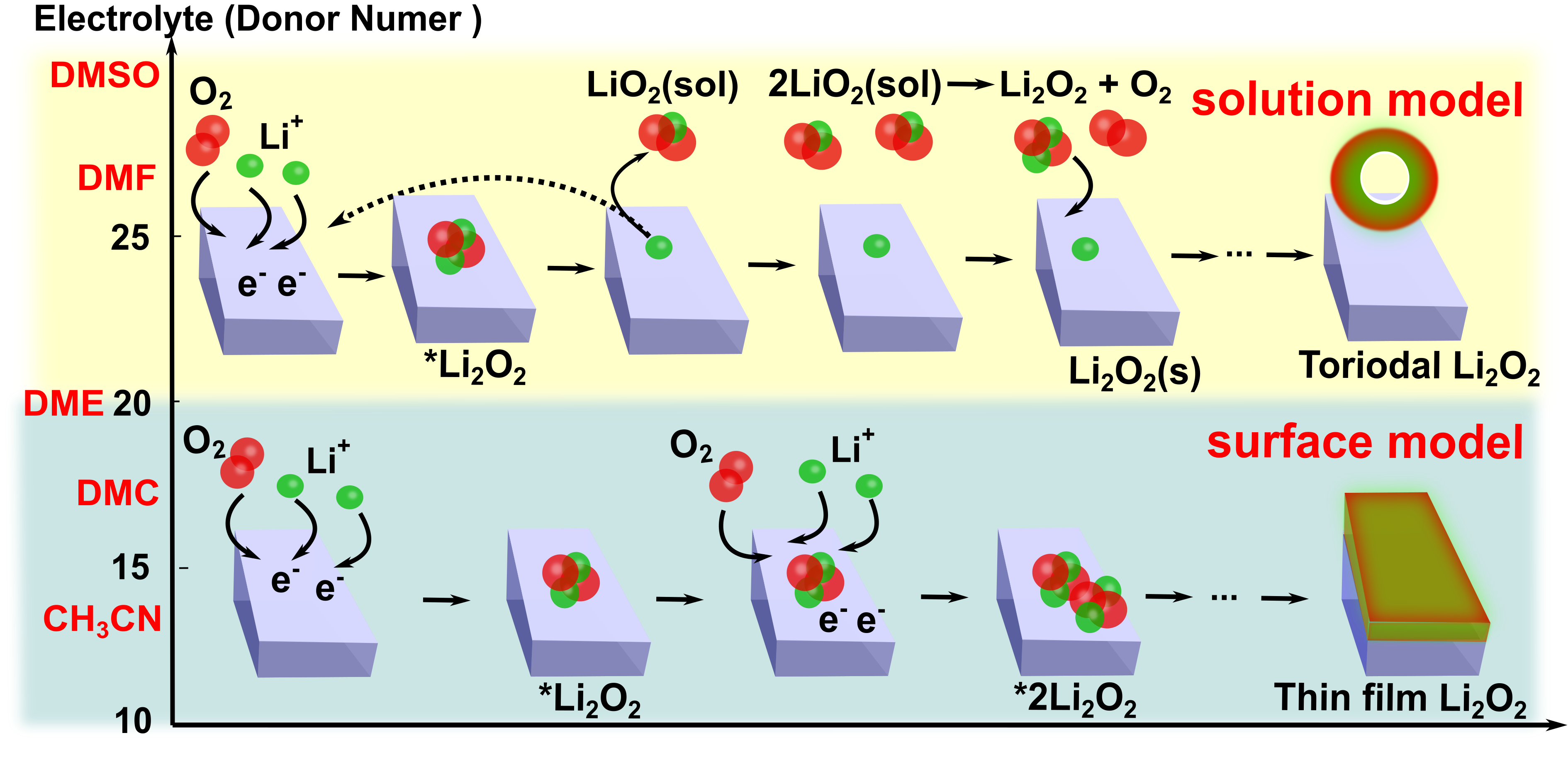
The Lithium-air batteries are found to offer extremely high theoretical energy density, as strong contenders for bringing conventional batteries into the next generation. Our ab initio thermodynamic calculations examined the electrode efficiency of TMPs (Mo3P), transition metal oxides (Co3O4), and metals (Au) by delivering the discharging/charging mechanisms and overpotentials (η). Afterwards, the discharging LiO2 monomer products was found favorable to be transported away from the electrode surface with the assistant of high donor number electrolytes, which delivers a solution model of discharging products growth mechanism and a toroidal morphology of ultimate discharging products. Therefore, the deactivation and passivation of the electrode due to the gradual covering of the surface by discharge products can be avoided. The two metrics of electrolytes (donor number and HOMO–LUMO gap) are found to be inversely linearly correlated, demonstrating the long-term activity and sustainable low overpotentials of electrodes strongly depend on the electron donor ability of aprotic solvents in the electrolytes.
Keywords: Interfacial Electrochemistry; Electrode Passivation; Aprotic Solvent (Donor Number); Ab Initio Molecular Dynamics Simulations
Publications:
Zhen Jiang, and Andrew M. Rappe J. Am. Chem. Soc. 144(48), 22150-22158 (2022). (Cover Art Invitation) [DOI]
Zhen Jiang, and Andrew M. Rappe J. Phys. Chem. C 126(25), 10266-10272 (2022). [DOI]
Zhen Jiang, and Andrew M. Rappe J. Phys. Chem. C 125(40), 21873-21881 (2021). [DOI]
Alireza Kondori, Zhen Jiang, Mohammadreza Esmaeilirad, Arvin Kakekhani, Kamil Kucuk, Pablo Navarro Munoz Delgado, Sadaf Maghsoudipour, John Hayes, Christopher S Johnson, Carlo U Segre, Reza Shahbazian-Yassar, Andrew M. Rappe, and Mohammad Asadi Adv. Mater. 2004028 (2020) [DOI]
